In the rapidly evolving landscape of energy storage technologies, certain compounds stand out for their transformative potential. Among these, fluoroethylene carbonate (FEC) has emerged as a true game-changer. This unassuming chemical compound is revolutionizing battery performance, particularly in lithium-ion batteries that power everything from our smartphones to electric vehicles and renewable energy storage systems.
But what exactly makes fluoroethylene carbonate so special? Why are researchers, battery manufacturers, and energy companies investing significant resources into understanding and implementing this compound? And how might it shape our energy future?
In this comprehensive exploration, we’ll dive into the world of fluoroethylene carbonate, examining its properties, applications, benefits, and the challenges that come with its implementation. Whether you’re a battery enthusiast, an energy professional, or simply curious about the technologies shaping our sustainable future, this guide will provide valuable insights into one of the most promising compounds in modern energy storage.
The Basics: What Is Fluoroethylene Carbonate?
Before we dive into the revolutionary applications of fluoroethylene carbonate, let’s establish a clear understanding of what this compound actually is.
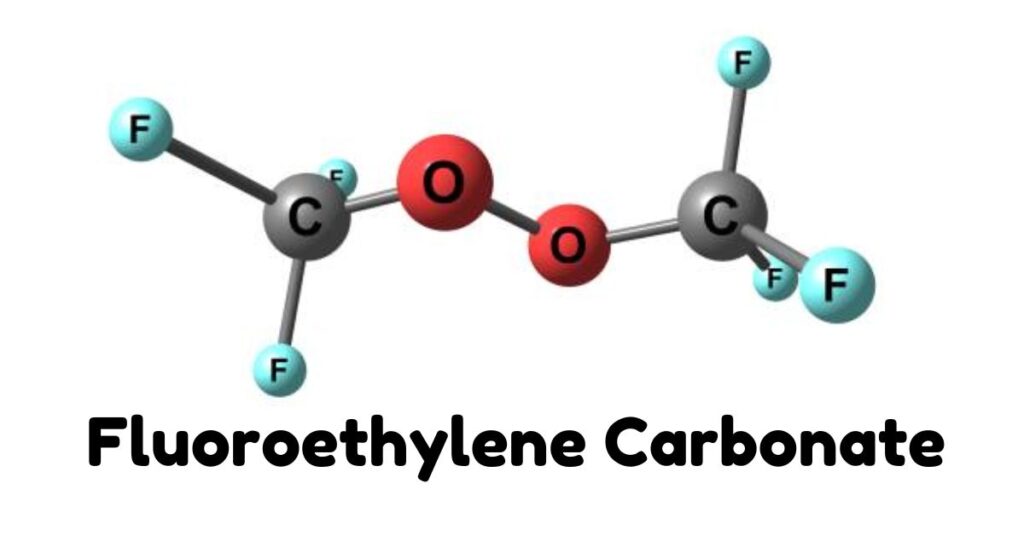
Fluoroethylene carbonate, often abbreviated as FEC, is an organic compound with the chemical formula C₃H₃FO₃. Structurally, it’s a derivative of ethylene carbonate where one hydrogen atom has been replaced by a fluorine atom. This seemingly minor modification significantly alters the compound’s properties and behavior, especially in battery applications.
FEC appears as a colorless liquid at room temperature, with a slightly higher viscosity than water. It has a relatively high boiling point and good thermal stability, which are crucial factors for its application in battery systems that can generate considerable heat during operation.
The introduction of the fluorine atom into the molecular structure gives FEC unique chemical properties, particularly in terms of how it interacts with electrode surfaces in batteries. This interaction fundamentally changes the performance characteristics of battery systems, which we’ll explore in detail in the following sections.
Chemical Properties That Make FEC Special
The magic of fluoroethylene carbonate lies in its unique chemical properties:
- Enhanced Stability: The carbon-fluorine bond is one of the strongest bonds in organic chemistry, contributing to FEC’s impressive stability even under challenging battery conditions.
- Excellent Solubility: FEC dissolves well in conventional battery electrolytes, making it easy to incorporate into existing battery formulations.
- Surface Film Formation: Perhaps most importantly, FEC has the remarkable ability to form stable, protective films on electrode surfaces through decomposition reactions.
- Low Reactivity: Despite its ability to form protective films, FEC exhibits low reactivity with other battery components, reducing unwanted side reactions.
These properties combine to make fluoroethylene carbonate an exceptionally valuable compound in modern energy storage technologies, particularly in lithium-ion batteries where electrode stability and longevity are persistent challenges.
How Fluoroethylene Carbonate Transforms Battery Performance
The energy storage industry is constantly searching for ways to improve battery performance across multiple parameters: energy density, power capability, cycle life, safety, and cost. Fluoroethylene carbonate addresses several of these aspects simultaneously, which explains its growing prominence in battery research and manufacturing.
The Solid Electrolyte Interphase (SEI) Revolution
The most significant contribution of fluoroethylene carbonate to battery technology involves the formation of the solid electrolyte interphase (SEI) layer. The SEI is a thin film that forms on the electrode surfaces during the initial charging cycles of a battery.
When fluoroethylene carbonate is included in the electrolyte formulation, it participates in the formation of this critical layer, fundamentally changing its properties:
- Improved Uniformity: FEC helps create a more uniform and continuous SEI layer, reducing “hot spots” where degradation can accelerate.
- Enhanced Mechanical Properties: The SEI formed with FEC contribution tends to be more flexible and resistant to cracking, even during volume changes in the electrode materials.
- Superior Chemical Stability: The FEC-influenced SEI resists dissolution and continuous reformation, preserving the electrode material and electrolyte.
- Better Ionic Conductivity: Despite forming a protective layer, the FEC-enhanced SEI maintains excellent lithium-ion conductivity, ensuring that battery performance isn’t compromised.
These improvements to the SEI layer translate directly into better battery performance, particularly in terms of cycle life and capacity retention.
Enabling Silicon Anodes for Higher Energy Density
One of the most exciting applications of fluoroethylene carbonate is its ability to enable the use of silicon anodes in lithium-ion batteries. Silicon offers a theoretical capacity nearly ten times higher than traditional graphite anodes, potentially allowing for much higher energy density batteries.
However, silicon faces a major challenge: it undergoes massive volume changes (up to 300%) during charging and discharging. These volume changes typically lead to electrode pulverization, SEI breakdown, and rapid capacity loss.
This is where fluoroethylene carbonate comes in. When used as an electrolyte additive, FEC forms a remarkably stable SEI on silicon surfaces that can withstand the extreme volume changes. This protective layer prevents continuous electrolyte decomposition and helps maintain electrical connectivity within the electrode.
Many researchers now consider fluoroethylene carbonate to be essential for practical silicon-based anodes, making it a key enabler for the next generation of high-energy-density batteries.
Applications Across the Energy Storage Spectrum
Fluoroethylene carbonate’s benefits extend across various battery technologies and applications. Let’s explore some of the most important areas where FEC is making a significant impact.
Electric Vehicle Batteries
The electric vehicle (EV) revolution demands batteries with higher energy density, faster charging capabilities, longer lifespans, and improved safety profiles. Fluoroethylene carbonate addresses several of these requirements:
- Extended Range: By enabling silicon-based or silicon-composite anodes, FEC helps increase energy density, potentially extending EV range.
- Longer Battery Life: The improved SEI formation leads to better cycle life, meaning EV batteries can last longer before needing replacement.
- Fast-Charging Capability: FEC-enhanced batteries often show improved rate capability, supporting faster charging without excessive degradation.
- Cold Weather Performance: Some studies suggest that FEC can improve low-temperature performance, addressing one of the key challenges for EVs in colder climates.
Major EV manufacturers are increasingly incorporating fluoroethylene carbonate into their battery formulations, recognizing its potential to address multiple challenges simultaneously.
Consumer Electronics
While EVs often get the spotlight in battery discussions, consumer electronics represent another massive market for advanced battery technologies. Here, fluoroethylene carbonate offers several compelling benefits:
- Longer Device Lifespan: The improved cycle life translates into devices that can go through more charge-discharge cycles before battery degradation becomes noticeable.
- Higher Energy Density: With FEC enabling silicon-containing anodes, manufacturers can either make devices with longer battery life or reduce battery size while maintaining the same performance.
- Enhanced Safety: The more stable SEI layer can help reduce the risk of thermal runaway and other safety incidents.
From smartphones and laptops to wearable devices and wireless earbuds, fluoroethylene carbonate is helping address the perpetual consumer demand for better battery performance in smaller packages.
Grid-Scale Energy Storage
As renewable energy sources like wind and solar provide an increasing share of our electricity, grid-scale energy storage becomes essential to balance supply and demand. Here too, fluoroethylene carbonate has a role to play:
- Extended Cycle Life: Grid storage batteries need to last through thousands of cycles to be economically viable. FEC’s ability to improve cycle life directly impacts the economic equation.
- Improved Calendar Life: Grid batteries may sit partially charged for extended periods. FEC helps reduce the calendar aging that can occur during these periods.
- Cost Efficiency: By extending battery life and improving performance, FEC can help reduce the levelized cost of storage, making renewable energy integration more economical.
While lead-acid, flow batteries, and other technologies compete in the grid storage space, lithium-ion batteries with advanced electrolyte formulations containing fluoroethylene carbonate are gaining market share due to their improving performance and decreasing costs.
Manufacturing and Supply Chain Considerations
The growing importance of fluoroethylene carbonate in energy storage technologies naturally raises questions about its production, supply chain, and economic factors.
Production Methods and Scalability
The commercial production of fluoroethylene carbonate typically involves the reaction of ethylene carbonate with electrophilic fluorinating agents. Several methods exist, each with different efficiencies, costs, and environmental impacts:
- Direct Fluorination: This approach uses fluorine gas or other direct fluorinating agents to introduce the fluorine atom into ethylene carbonate.
- Electrochemical Fluorination: Some newer methods use electrochemical techniques to accomplish the fluorination step.
- Catalytic Processes: Various catalysts can be employed to improve the efficiency and selectivity of the fluorination reaction.
As demand for fluoroethylene carbonate grows, manufacturers are investing in scaling up production and improving efficiency. However, the production processes still face challenges in terms of cost, yield, and environmental impact.
Supply Chain Resilience
With fluoroethylene carbonate becoming increasingly critical to advanced battery performance, supply chain considerations take on strategic importance:
- Geographical Concentration: Currently, production is concentrated in a few countries, raising concerns about supply chain resilience.
- Raw Material Dependencies: The production of FEC requires specific chemical precursors, some of which have their own supply chain challenges.
- Quality Control: As with any battery material, consistent quality is essential for reliable performance, requiring sophisticated quality control measures.
Battery manufacturers and energy companies are increasingly looking to secure reliable supplies of high-quality fluoroethylene carbonate as part of their strategic planning.
Environmental Considerations and Sustainability
As we embrace technologies that enable the transition to cleaner energy, it’s important to consider the environmental impact of the materials involved, including fluoroethylene carbonate.
Environmental Impact Assessment
The environmental profile of fluoroethylene carbonate should be considered across its entire lifecycle:
- Production: The synthesis of FEC can involve energy-intensive processes and potentially hazardous chemicals, though manufacturers are working to improve efficiency and reduce environmental impact.
- Use Phase: During battery operation, FEC contributes to longer battery life, which can reduce the environmental impact per unit of energy stored and delivered.
- End-of-Life: Battery recycling processes need to account for the presence of fluorinated compounds like FEC in electrolyte formulations.
Research into greener production methods for fluoroethylene carbonate is ongoing, with efforts focused on reducing energy consumption, minimizing waste, and finding safer alternatives to some of the reagents currently used.
Recycling Challenges and Opportunities
As batteries containing fluoroethylene carbonate reach the end of their useful life, recycling becomes an important consideration:
- Recovery Methods: Current battery recycling processes primarily focus on recovering valuable metals like cobalt, nickel, and lithium, with less attention paid to electrolyte components.
- Chemical Transformations: During battery usage and especially during recycling processes, FEC may undergo chemical transformations that need to be understood and managed.
- Closed-Loop Systems: Ideally, future recycling technologies would recover and regenerate fluoroethylene carbonate as part of a closed-loop system for battery materials.
The recycling infrastructure for advanced batteries is still developing, and the inclusion of fluorinated compounds like FEC presents both challenges and opportunities for creating truly sustainable energy storage solutions.
Future Directions for Fluoroethylene Carbonate Research
The story of fluoroethylene carbonate in energy storage is still unfolding, with numerous research directions being actively pursued.
Beyond Lithium-Ion: Next-Generation Battery Technologies
While much of the current focus is on lithium-ion batteries, researchers are exploring FEC’s potential contributions to other battery chemistries:
- Lithium-Sulfur Batteries: These promise even higher energy density than current lithium-ion batteries, and FEC may help address some of their stability challenges.
- Sodium-Ion Batteries: As a potentially lower-cost alternative to lithium-ion, sodium-ion batteries might also benefit from FEC-enhanced electrolytes.
- Solid-State Batteries: Even in solid-state configurations, interface stability remains crucial, and FEC-derived components may play a role.
The fundamental understanding gained from studying fluoroethylene carbonate in lithium-ion systems provides valuable insights that could accelerate the development of these next-generation technologies.
Molecular Engineering for Enhanced Performance
Scientists are also working on modified versions of fluoroethylene carbonate with tailored properties:
- Multi-Fluorinated Derivatives: Adding additional fluorine atoms to the structure may further enhance certain properties.
- Functional Group Modifications: Introducing other functional groups alongside the fluorine can create compounds with specialized characteristics.
- Co-Additives: Combinations of FEC with other electrolyte additives often show synergistic effects that exceed the benefits of either compound alone.
This molecular engineering approach aims to develop even more effective electrolyte systems tailored to specific battery requirements.
Practical Implications for Energy Users
For those who use and depend on energy storage technologies—which includes virtually everyone in the modern world—the advancements enabled by fluoroethylene carbonate translate into tangible benefits.
What This Means for Consumers
As batteries incorporating fluoroethylene carbonate become more common, consumers can expect:
- Longer-Lasting Devices: Smartphones, laptops, and other electronics may maintain their original battery performance for longer periods.
- Faster-Charging Options: Improved battery chemistry could enable faster charging without compromising battery longevity.
- More Affordable Electric Vehicles: As battery performance improves and costs decrease, EVs may reach price parity with conventional vehicles sooner.
- Reduced Environmental Impact: Longer-lasting batteries mean fewer replacements and less material consumption over time.
These improvements may not always be explicitly marketed—you won’t likely see “contains fluoroethylene carbonate” on product packaging—but they contribute to the steady improvement in battery-powered devices that consumers have come to expect.
Industrial and Utility-Scale Impacts
At larger scales, the benefits become even more significant:
- More Economical Renewable Integration: Better energy storage makes wind and solar power more practical and economical to integrate into the grid.
- Enhanced Grid Resilience: Advanced batteries can provide backup power during outages and help stabilize the grid during demand fluctuations.
- Industrial Electrification: Improved batteries support the electrification of industrial processes that currently rely on fossil fuels.
As these technologies mature, they contribute to the broader transition to more sustainable energy systems.
Conclusion: Fluoroethylene Carbonate’s Pivotal Role in Our Energy Future
As we’ve explored throughout this article, fluoroethylene carbonate represents far more than just another chemical compound in the battery researcher’s toolkit. It stands as a critical enabler for multiple advancements in energy storage technology, with implications that ripple throughout our energy systems.
The ability of fluoroethylene carbonate to form superior protective interfaces on electrode surfaces addresses one of the fundamental challenges in battery technology: the gradual degradation that occurs at the electrode-electrolyte boundary. By solving this problem, FEC helps unlock the potential of high-capacity materials like silicon, extends the useful life of batteries across applications, and improves safety characteristics.
While challenges remain in terms of production scaling, cost reduction, and environmental considerations, the trajectory is clear: fluoroethylene carbonate and related compounds will play an increasingly important role in the battery technologies that power our transition to more sustainable energy systems.
As research continues and manufacturing processes mature, we can expect to see the benefits of fluoroethylene carbonate become more widespread—from the devices in our pockets to the vehicles in our driveways and the energy systems that power our communities.
The story of fluoroethylene carbonate reminds us that sometimes, seemingly small molecular modifications can lead to outsized impacts on technology and society. In the global effort to develop cleaner, more efficient energy storage, this unassuming compound has indeed proven to be a game-changer.
FAQs About Fluoroethylene Carbonate
What exactly is fluoroethylene carbonate?
Fluoroethylene carbonate (FEC) is an organic compound with the formula C₃H₃FO₃, structurally similar to ethylene carbonate but with one hydrogen atom replaced by a fluorine atom. This modification gives it unique properties that make it valuable as an electrolyte additive in lithium-ion batteries.
How does fluoroethylene carbonate improve battery performance?
FEC primarily improves battery performance by forming a more stable and effective solid electrolyte interphase (SEI) layer on electrode surfaces. This protective layer prevents continuous electrolyte decomposition, reduces unwanted side reactions, and helps maintain electrode integrity, especially during cycling.
Is fluoroethylene carbonate safe to use in consumer products?
When properly incorporated into battery systems, FEC is considered safe for consumer applications. In fact, it can enhance battery safety by improving thermal stability and reducing the risk of electrolyte decomposition. However, like many chemical compounds, pure FEC should be handled with appropriate safety measures during manufacturing.
Does using fluoroethylene carbonate make batteries more expensive?
While FEC itself adds some cost to battery production, the performance benefits it provides—particularly longer cycle life and enabling higher-capacity materials—can actually improve the overall economics of battery systems. As production scales up, the cost impact of FEC is expected to decrease further.
Can fluoroethylene carbonate help make electric vehicles more practical?
Yes, FEC contributes to making EVs more practical by enabling batteries with potentially higher energy density (longer range), better cycle life (longer battery lifespan), improved fast-charging capabilities, and enhanced low-temperature performance—all key factors in consumer adoption of electric vehicles.
How environmentally friendly is fluoroethylene carbonate?
The environmental profile of FEC is complex. While it contains fluorine, which raises some environmental concerns, its contribution to extending battery life and enabling more efficient energy storage systems provides significant environmental benefits. Research continues on greener production methods and effective recycling approaches.
Can I purchase batteries with fluoroethylene carbonate for my own use?
Many commercial lithium-ion batteries already incorporate FEC in their electrolyte formulations, though this isn’t typically advertised in consumer-facing materials. If you’re using modern smartphones, laptops, or electric vehicles, there’s a good chance your battery already benefits from FEC technology.
What’s the future of fluoroethylene carbonate in energy storage?
FEC is likely to remain important in lithium-ion batteries while also finding applications in next-generation technologies like lithium-sulfur, sodium-ion, and possibly even solid-state batteries. Research continues on modified versions with enhanced properties tailored to specific battery chemistries and applications.
How does fluoroethylene carbonate compare to other electrolyte additives?
FEC is often considered one of the most effective electrolyte additives, particularly for silicon-containing anodes. However, it’s increasingly used in combination with other additives in carefully designed electrolyte formulations that provide synergistic benefits across multiple performance parameters.
Will fluoroethylene carbonate help reduce our dependence on fossil fuels?
By improving the performance and economics of energy storage systems, FEC indirectly contributes to the broader transition away from fossil fuels. Better batteries make renewable energy more practical for grid applications and accelerate the electrification of transportation, both critical steps in reducing fossil fuel consumption.